
Resources
Publications and clinical cases presenting Seegnal value
Publications & Case Studies
Clinical Use Cases
-
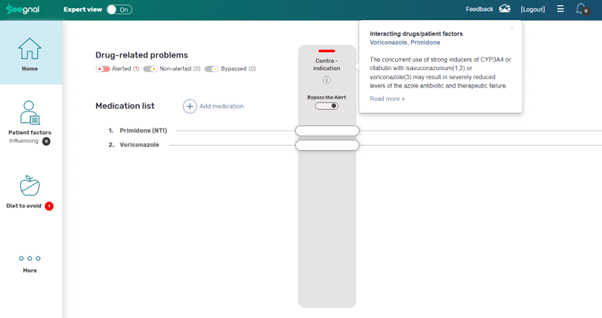
Reducing the risk for antifungal therapeutic failure in a critically ill patient
Setting: Hospital
Alert category: Drug-drug interaction
Case: A patient with hemato-oncologic disease and lung aspergillosis was prescribed the antifungal voriconazole, alongside their regular anti-tremor medication, primidone. This combination is not recommended because primidone can substantially lower voriconazole's blood levels and effectiveness, which may result in therapeutic failure.
Impact: Seegnal alerted the healthcare team, allowing proactive intervention to reduce the risk by withholding primidone and monitoring the voriconazole level
-
Reducing inappropriate use of domperidone in a pediatric department
Setting: Hospital
Alert category: Severe pediatric precaution
Case: In a pediatric department, a high rate of prescriptions for the prokinetic and antiemetic drug domperidone was found in children under 12 years old. Due to the potential risk of serious heart-related side effects and the lack of evidence supporting its effectiveness for gastroenteritis in children under 12, domperidone is no longer approved in this age group.
Impact: During the silent run and the first month after implementation, Seegnal's clinical team observed a high rate of alerts. They approached the head of the Pediatrics department, who issued a clarification to the medical staff. This led to an immediate and significant reduction in the domperidone prescription rate in children younger than 12 years, which has since remained consistently low.

-
Prevention of life-threatening hypersensitivity reaction
Setting: health maintenance organizations (HMO)
Alert category: Drug-Allergy
Case: A patient with a known severe allergic reaction to chlorpromazine (Stevens-Johnson syndrome (SJS)- a life-threatening allergic reaction affecting the skin and mucous membranes) was prescribed promethazine as a substitute, a drug with likely cross-sensitivity.
Impact: Seegnal triggered an alert for allergy cross-sensitivity, the error was thus discovered by the clinical pharmacist before drug administration, and a potentially fatal adverse drug effect was prevented.

-
Facilitating preventive measures to reduce tacrolimus toxicity
Setting: Hospital
Alert category: Drug-drug interaction
Case: A 56-year-old liver transplant patient, on tacrolimus 4 mg twice daily maintenance dose, was admitted with candida esophagitis, and fluconazole 400 mg daily was started. Concurrent administration of an azole antifungal may result in elevated levels and toxicity from tacrolimus.
Impact: Seegnal's alert prompted clinicians to review the therapy, discovering an excessively high tacrolimus blood concentration (38 ng/mL; therapeutic range, 5–15 ng/mL). The tacrolimus dose was reduced to 1 mg twice daily, and the patient was discharged with a two-week course of fluconazole, along with instructions to continue therapeutic drug monitoring and expect higher dose requirements after the conclusion of the fluconazole therapy.

-
Switch contraception to prevent potential loss of seizure control
Setting: health maintenance organizations (HMO)
Alert category: Drug-Drug Interaction
Case: A young woman recovering from brain tumor surgery, experiencing fatigue and nervousness as side effects of levetiracetam, was advised by her private neurologist to switch to lamotrigine. However, when her family physician at her HMO attempted to implement this change based on the specialist's recommendation, the Seegnal platform flagged a potentially severe interaction between lamotrigine and the Nuvaring® vaginal ring the patient was using. Estrogen-containing contraceptives like Nuvaring® can reduce lamotrigine levels, increasing the risk of seizures.
Impact: Seegnal notified the physician and enabled them to explore safer contraceptive alternatives, such as copper IUDs and etonogestrel implants, as recommended by the guidelines, without risking the patient by lowering the threshold for convulsions.

-
Support of institutional-wide project aimed at reducing inappropriate use of nitrofurantoin
Setting: health maintenance organizations (HMO)
Alert category: Renal dose adjustments or contraindication
Case: Prior to the implementation of Seegnal, a high rate of nitrofurantoin use was identified among elderly patients with decreased renal function. As a result, an intervention to reduce this inappropriate use was planned and executed by the institute's clinical pharmacists, coinciding with the launch of Seegnal as a clinical decision support system.
Impact: Seegnal alerts in this HMO integration disrupt the workflow, requiring physicians to either resolve the issue or bypass it. Hence, Seegnal supported the clinical pharmacist's efforts and helped facilitate the doctors' acceptance of the recommendations in the project.






